bottled water container microbiology testing|Testing microbiological : makers The consumption of bottled water containing certain bacteria or groups of bacteria and the implications for public health. [Google Scholar] Reyes M.I., Pérez C.M., Negrón E.L. Microbiological assessment of house and imported bottled water by comparison of bacterial endotoxin concentration, heterotrophic plate count, and fecal coliform count . O ataque em “Portal Zacarias atacados em live” serve como um lembrete da necessidade de manter a segurança e a proteção, especialmente em ambientes públicos e nas plataformas de mídia social onde a interação online pode levar a desentendimentos perigosos. A hashtag “portal zacarias atacados em live” continua a gerar discussões .
{plog:ftitle_list}
In this powerful video, join us as we dive into the remarkable journey of Gracie Bon, a trailblazing plus-size model and advocate for body positivity. From a.
Testing microbiological
As the world-premier provider of innovative drinking water microbiology test kits, IDEXX is known for its breakthrough products. IDEXX provides easy, rapid, accurate, and cost-effective water testing solutions.Sometimes, although the bottled water or beverages meet the quality requirements, still, they could be responsible by some water- or food-borne diseases. This chapter presents the main .
refractometer for dexcool
Sampling and Testing of Purified Water in Microbiology 1.0 PURPOSE: To lay down the procedure for Sampling and Testing of Purified Water. 2.0 SCOPE: This Standard Operating Procedure is applicable to the .The consumption of bottled water containing certain bacteria or groups of bacteria and the implications for public health. [Google Scholar] Reyes M.I., Pérez C.M., Negrón E.L. Microbiological assessment of house and imported bottled water by comparison of bacterial endotoxin concentration, heterotrophic plate count, and fecal coliform count .Discover our wide range of plastic water bottles for sampling analysis in laboratories. Available in many different sizes from 125ml to 1L , shapes from wide to narrow necks. . Brewery Gravity Testing; Microbiology Media . All .
Bottled Water or Ice Microbiology, Requisition 411 Instructions To document information concerning collection, testing, and reporting of results for . 1.2 Product water testing: . bottled water product container, in accordance with the instructions. 2. Barcode labels which specify the company name and permit number areBottled Water or Ice Microbiology, Requisition 411 Instructions To document information concerning collection, testing, and reporting of results for . 1.2 Product water testing: . bottled water product container, in accordance with the instructions. 2. Barcode labels which specify the company name and permit number are
Understanding container closure integrity systems, reviewing past observations, and following the regulations and guidance documents are excellent ways to establish a compliant container closure integrity assay. This article describes recent changes to the United States Pharmacopeia (USP) , guidance documents, regulatory observations, .Do not touch the inside of the lid or the rim of the sample container. Tightly cap the bottle with the provided lid and place in the Styrofoam packing material. Complete the form and wrap it around the Styrofoam. Pool, beach and surface water samples are sampled by dipping the sampling bottle into the water using a forward motion.IDEXX entered the water-testing market in 1993 with Colilert, its popular coliform and E.coli water test, which is included in Standard Methods of Examination of Water and Wastewater, 22nd edition. IDEXX’s sales, customer service, and technical support teams serve customers in over 170 countries.
Bottled Water or Ice Microbiology, Requisition 411 Instructions To document information concerning collection, testing, and reporting of results for . 1.2 Product water testing: . bottled water product container, in accordance with the instructions. 2. Barcode labels which specify the company name and permit number are Let water run for an additional 2-3 minutes after treatment. If sampling from a hot/cold tap, run hot water for 2 minutes then cold water for 2-3 minutes and collect samples as above. Tightly cap the bottle. Reservoirs (e.g. Tanks) Hold the closed bottle near its base and plunge it below the surface.200 bottled water microbiology ofthesemycobacteria( M.Chelonae , M.flavescens )areopportunisticpathogensthat may be harmful to immunologically deficient consumers (Papapetropoulou et al.,Principles apply to containers for API, bulk, intermediates. Packaged headspace. Air or nonreactive gases. At specified water vapor content. At ambient or sub-ambient pressures. Package (aka Container-closure): Primary package components. In direct product contact (or may be) Secondary package components critical for ensuring package assembly . E.g
In a trailblazing study, researchers have discovered bottled water sold in stores can contain 10 to 100 times more bits of plastic than previously estimated — nanoparticles so infinitesimally . A sample of 500 ml will be more than enough for this lab. If you cannot, we will have water samples in the lab. In Ontario, drinking water is regulated provincially by the Ministry of the Environment and Climate Change (MoECC), while bottled water is regulated federally by the Food and Drug Act.Drinking Water Testing Services. Accredited by the Washington State Department of Ecology, Sound Microbiology Laboratory performs quality bacteriological Drinking Water Testing and Analysis (including Well .accessible. Do not sample from a garden hose or from a container such as a water bottle. 6. If the water sample is to be taken from a distribution – system tap without attachments, select a tap that is supplying water from a service pipe directly connected with the main, and is not, for example, served from a cistern or storage tank. 7.
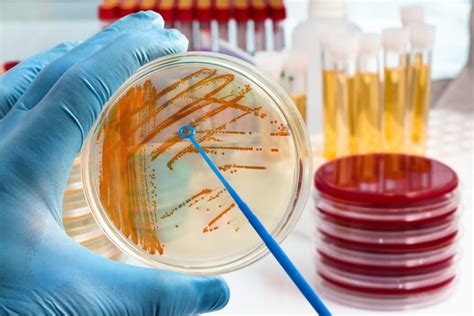
Molecular and Food Microbiology Environmental and Water Microbiology 3-in-1 and 4-in-1 Swab Testing. Plant and Crop Testing. . Hill Labs can utilise the very latest technologies to conduct water testing including: bottled water testing, swimming pool and spa pool water testing, and winery water testing.The principal microbiological contaminants found in drinking water of the United States are bacteria, viruses, and pathogenic protozoa. Each is considered in a separate section of this chapter. Helminths are included along with the protozoa. Little information is available on mycoplasma, pathogenic yeast, and pathogenic fungi in drinking water. Microbiological .9020 A. Introduction 1. General Considerations A quality management system (QS) for microbiological analyses establishes a quality assurance (QA) policy or program and quality control (QC) operational techniques and practices. These are designed to • substantiate the validity of analytical processes and data; • ensure compliance with regulatory requirements, • .
The FDA describes bottled water as water that’s intended for human consumption and sealed in bottles or other containers with no added ingredients, except that it may contain safe and suitable .of Bottled Water (10NS2) DeceMber 2011 MicrobiologY. Microbiological Safety of Bottled Water (10NS2) . water, spring water and other water, supplied in bottles or containers, from bottled water manufacturers and retail outlets throughout the Republic of Ireland. . Chi square (X2) analysis or Fisher’s Exact Test was performed using SPSS .Buy and find information on Corning coliform water test containers from Sigma-Aldrich.com, product number CLS1700100 . 120 mL polypropylene bottle, graduated, sterile, 100/cs. Synonym(s): sterile sample bottles. Sign In to View Organizational & Contract Pricing. Select a Size. 100 EA. 8.00. CLS1700100-100EA. TVC = Total Viable Count. (Total Bacterial Count + Total fungal Count) Note: For Raw water/Dm Water dilute the sample 10 times i.e. 1:10 and take 1 ml of diluted sample for analysis. 2.1 Total Viable Count (TVC) Test for Pure steam/DM Water/Purified water/water for injection: For Raw Water / DM Water: (Pour plate method)
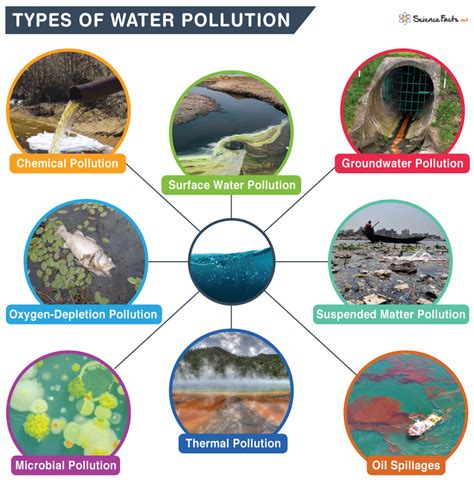
The “Test Portion” •Laboratory sample preparation => “test portion” –“analytical unit” or “analytical portion” –Definition: the part of the “sample” that is actually tested by the laboratory •The test portion determines the theoretical (i.e., best possible) sensitivity of the test –e.g., 1 cell/test portion Water activity, or Aw, is a measure of available water and when applied to a non-sterile pharmaceutical drug product is a critical physical attribute that determines whether the product will . 1. INTRODUCTION. Water pollution is a major global problem which has been the leading cause of morbidity and mortality worldwide and requires evaluation and revision of bottled water policy (Gautam et al., 2021).Almost 90% of child deaths are due to dehydration resulting from diarrheal disease as the sporadic, endemic, and epidemic cases of diarrhea are . More emphasis is placed on the microbiological quality of bottled water than hazards from toxic chemicals. The origin of these chemicals is the source water, introduction during bottling, or migration from the bottle to the water. Regulations concerning bottled water appear to be generally much stricter in Europe than in the United States.
Main Microbiological Pollutants of Bottled Waters and Beverages
refractometer for dogs
Major cellular providers are creating unified customer identifie.
bottled water container microbiology testing|Testing microbiological